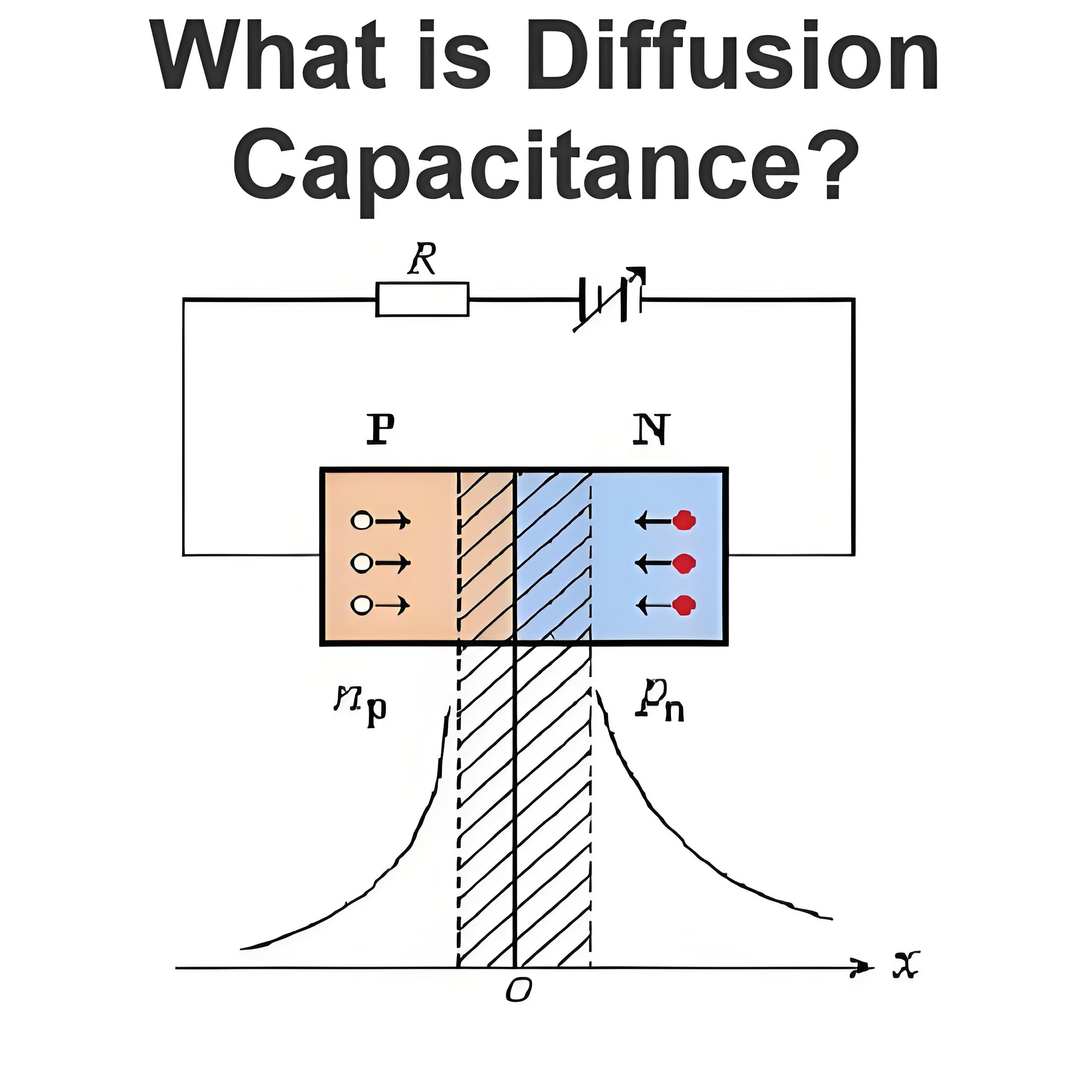
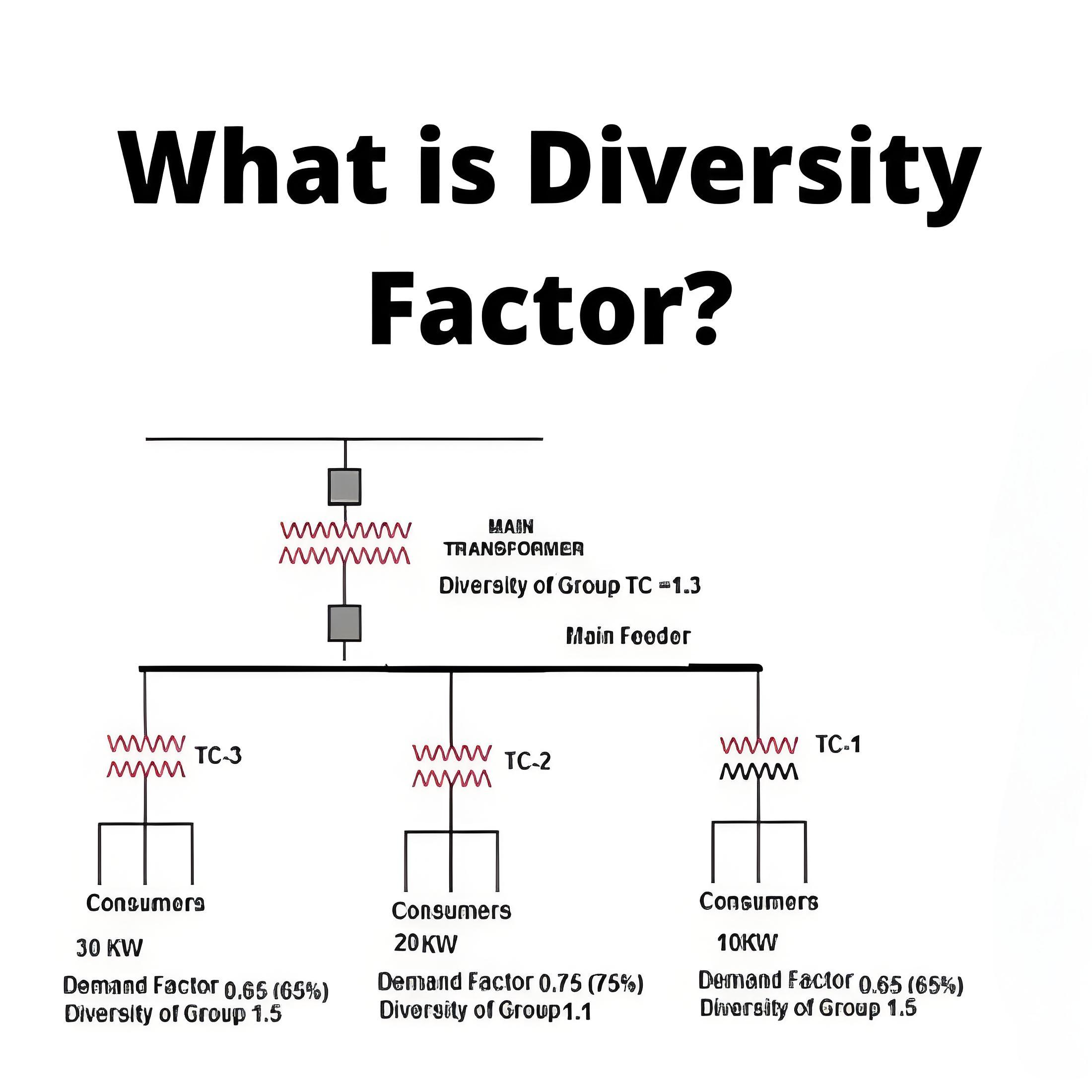
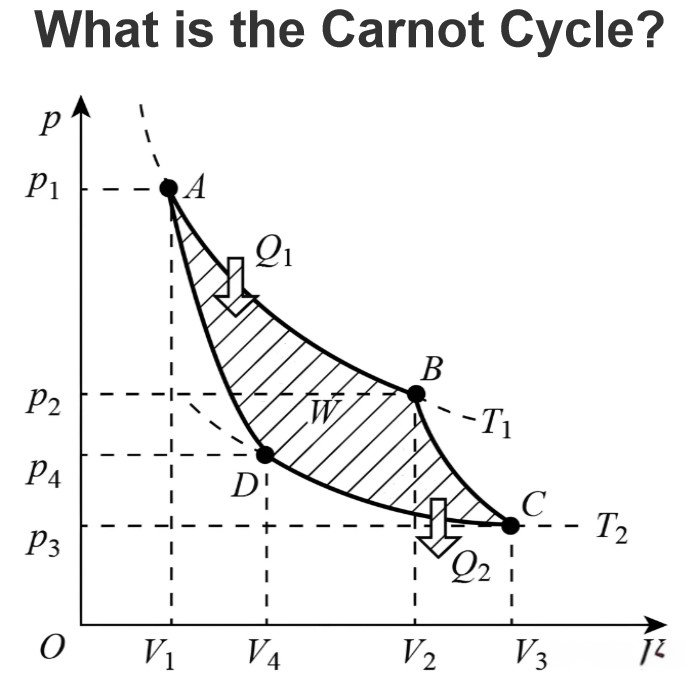
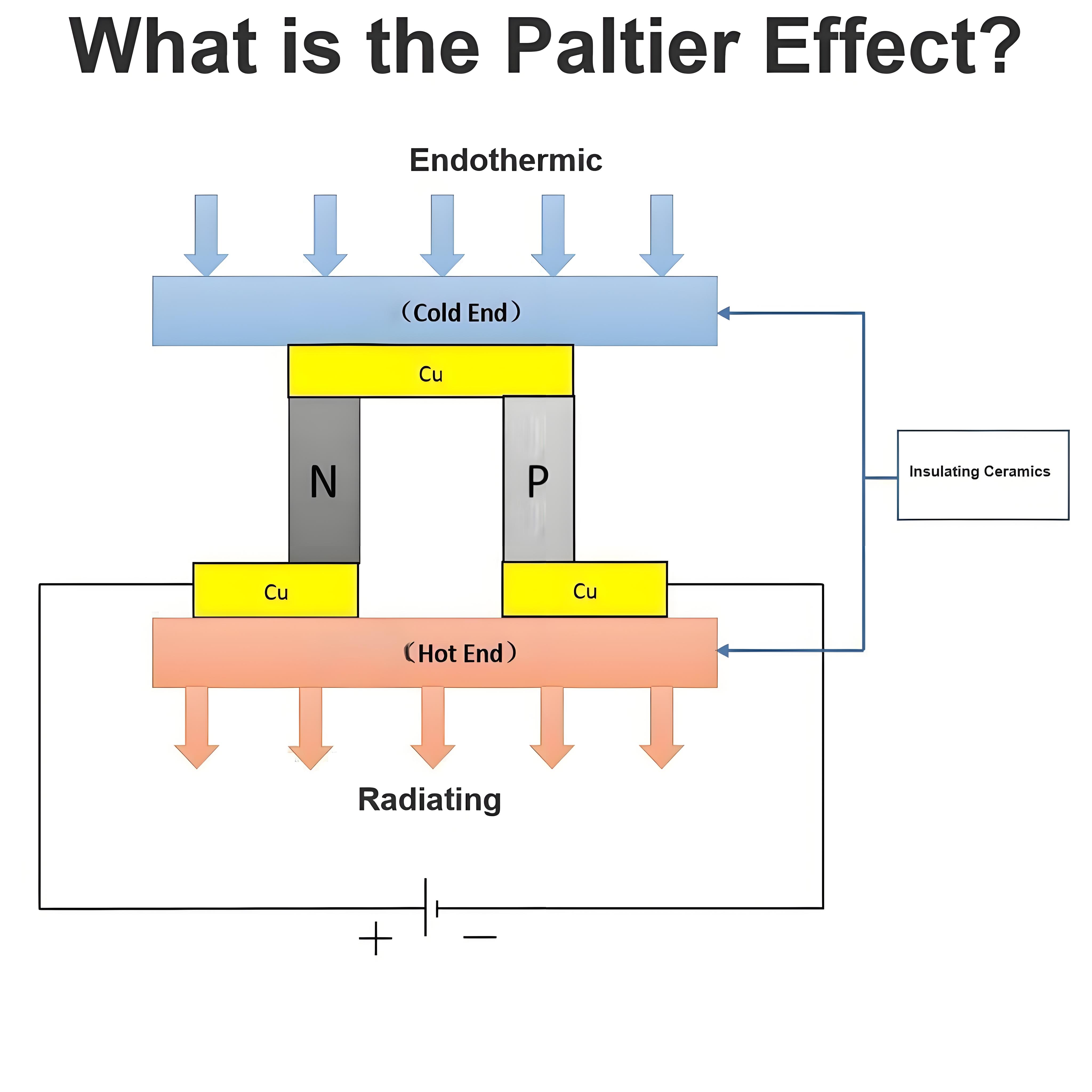
How Dose a Battery Work?
How Dose a Battery Work?
Battery Working Principle Definition
A battery works by converting chemical energy into electrical energy through the oxidation and reduction reactions of an electrolyte with metals.
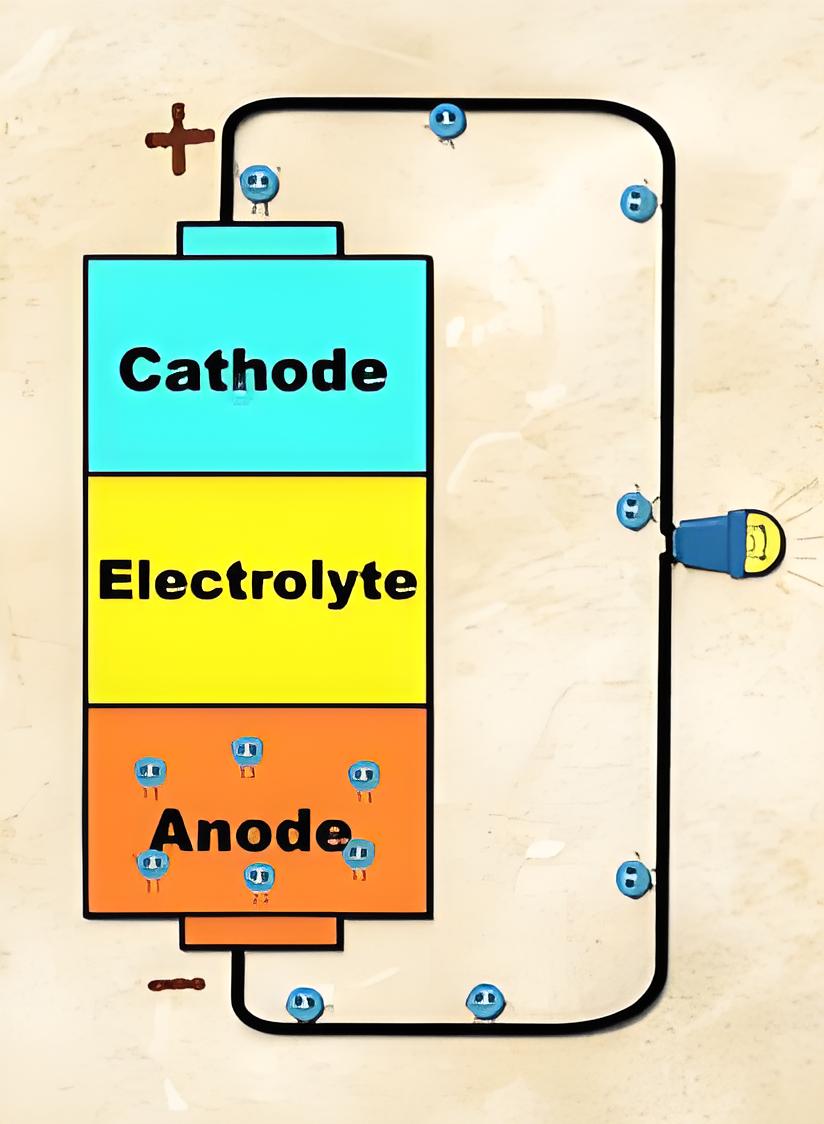
Electrodes and Electrolyte
The battery uses two dissimilar metals (electrodes) and an electrolyte to create a potential difference, with the cathode being the negative terminal and the anode the positive terminal.
Electron Affinity
Electron affinity determines which metal in the electrolyte will gain or lose electrons, influencing the direction of the current.
Voltaic Cell Example
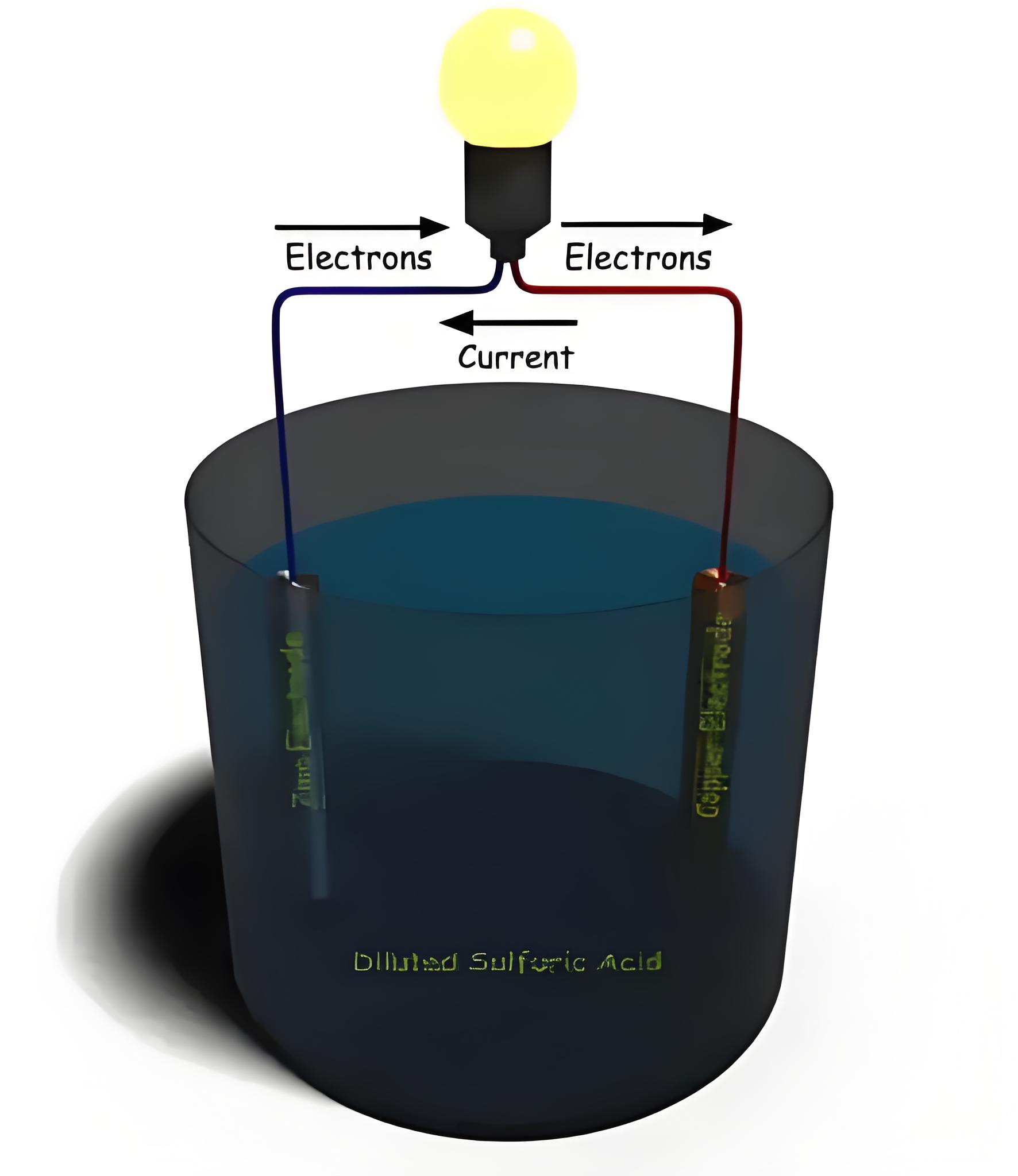
A simple voltaic cell uses zinc and copper electrodes in diluted sulfuric acid to generate electricity, illustrating the basic battery working principle.
Historical Development
The evolution of batteries from ancient Parthian batteries to modern lead-acid batteries shows advancements in creating stable and rechargeable power sources.
Welcome to our electricity community! Established to facilitate the exchange and cooperation in the electricity industry and bridge professionals, enthusiasts, and related enterprises.