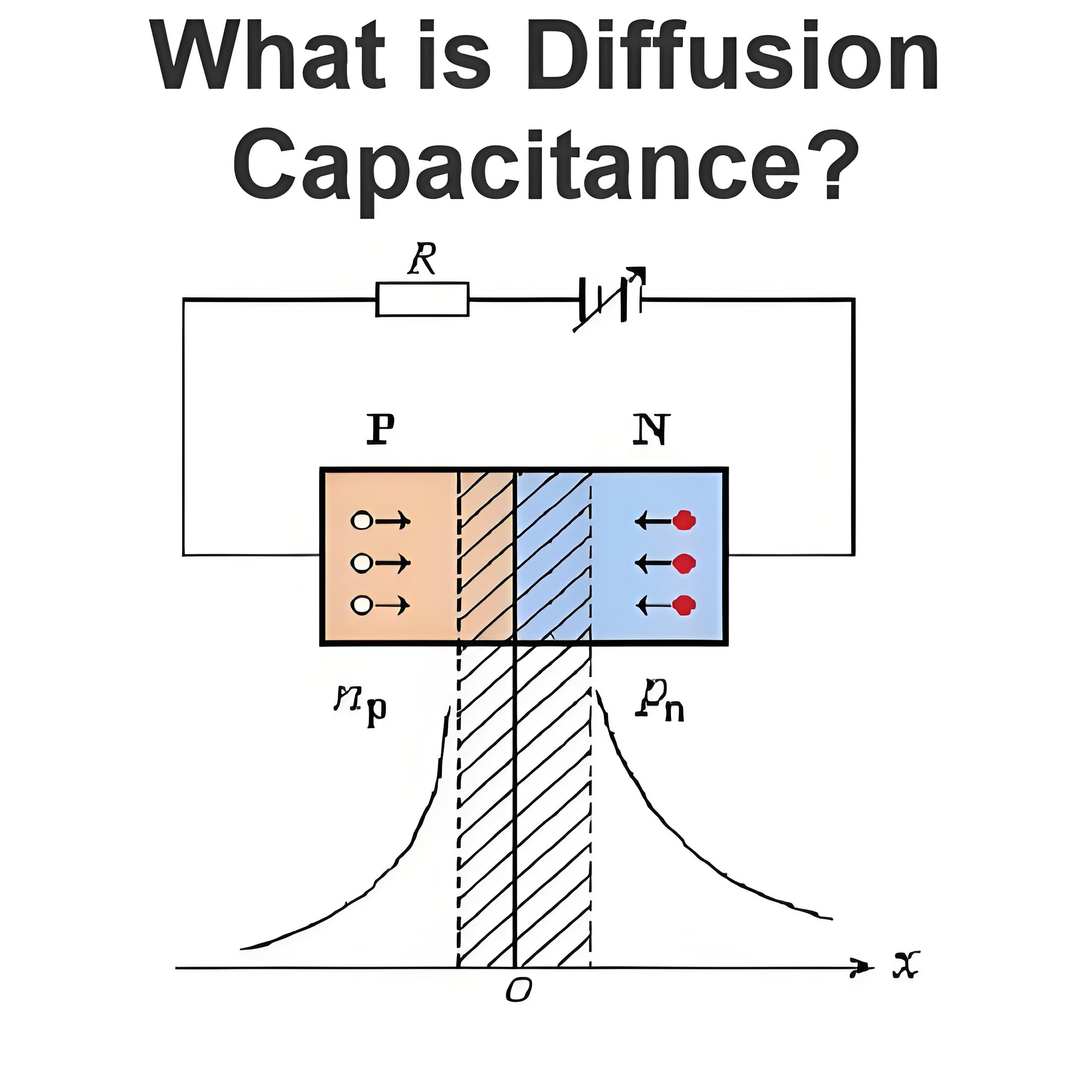
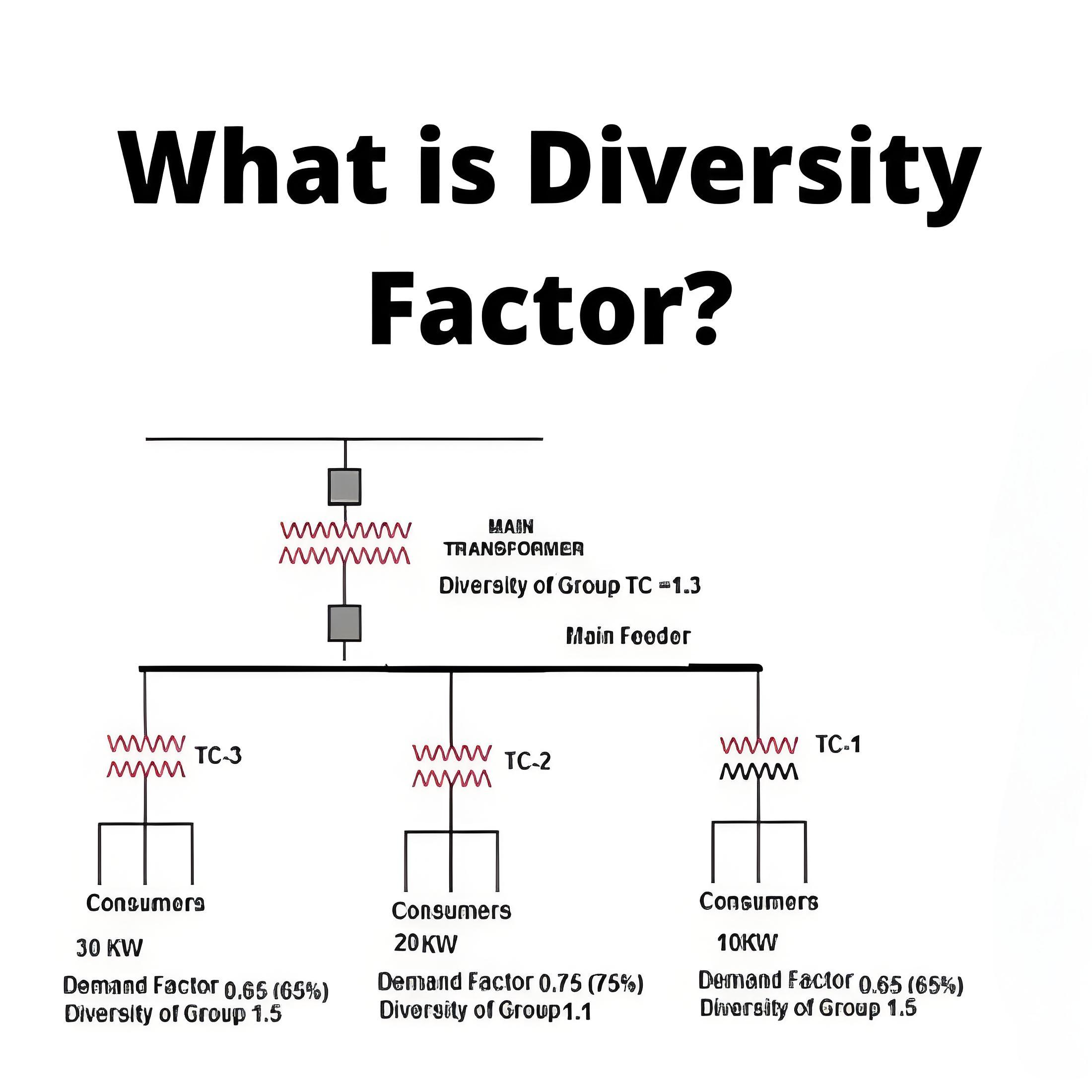
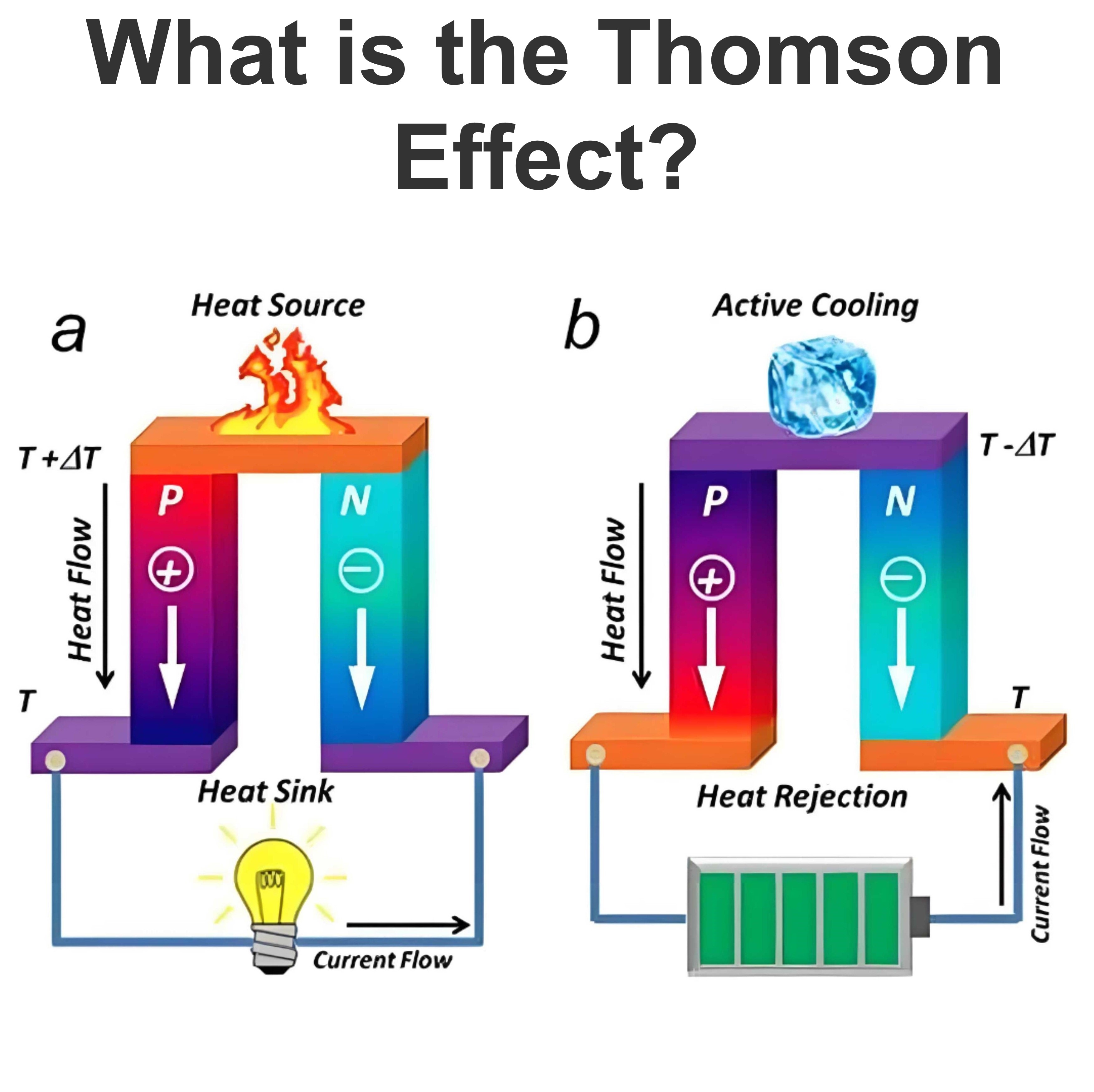
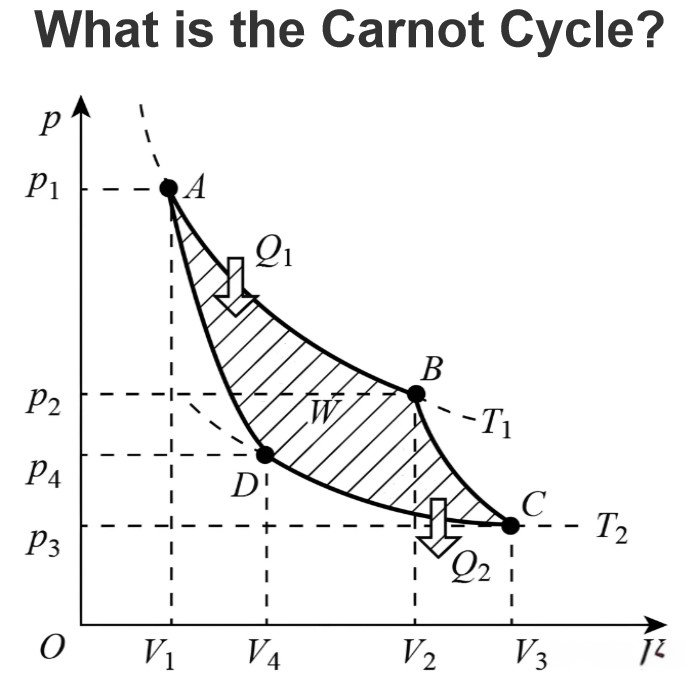
What is a Daniell Cell ?
What is a Daniell Cell ?
Daniell Cell Definition
A Daniell Cell is defined as an improved version of the Voltaic Cell that prevents polarization by converting chemical energy into electrical energy.
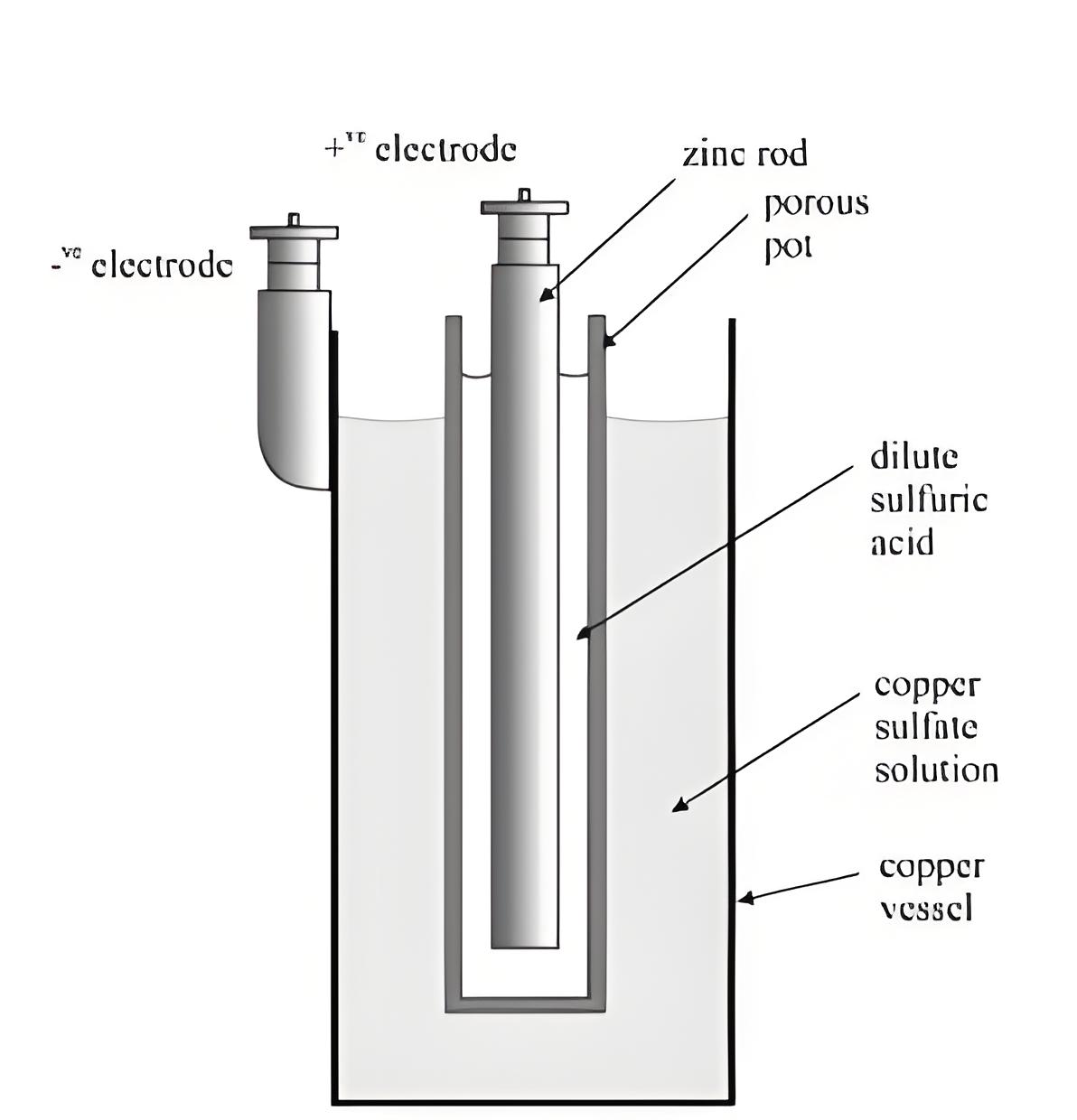
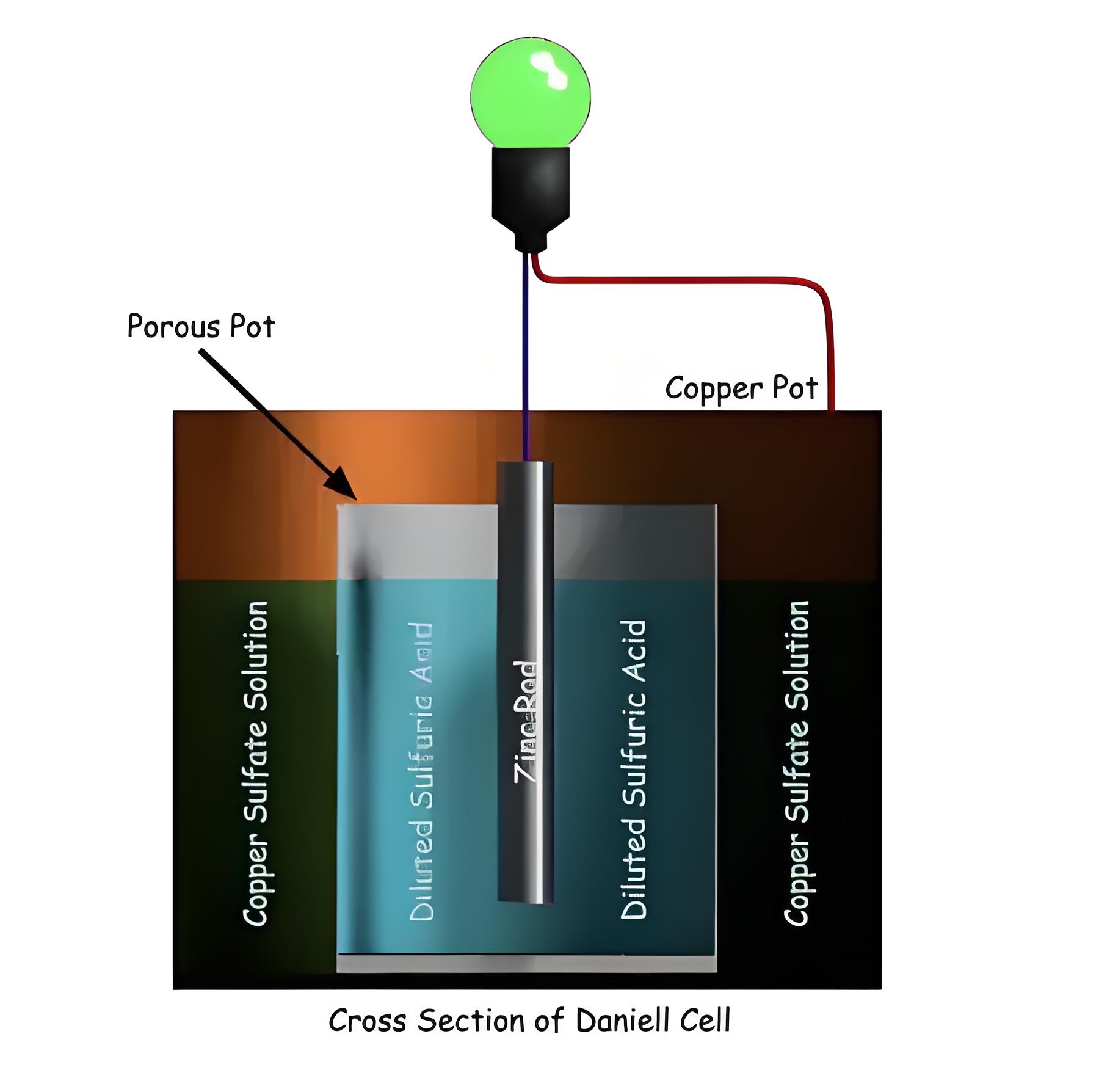
Construction of Daniell Cell
The cell consists of a copper container with copper sulfate solution and a porous pot filled with diluted sulfuric acid containing a zinc rod.
Oxidation and Reduction
Oxidation occurs at the zinc rod (cathode), forming zinc sulfate, while reduction occurs at the copper container (anode), depositing copper.
Ion Movement
Hydrogen ions move through the porous pot to form sulfuric acid in the copper sulfate solution, facilitating continuous cell reactions.
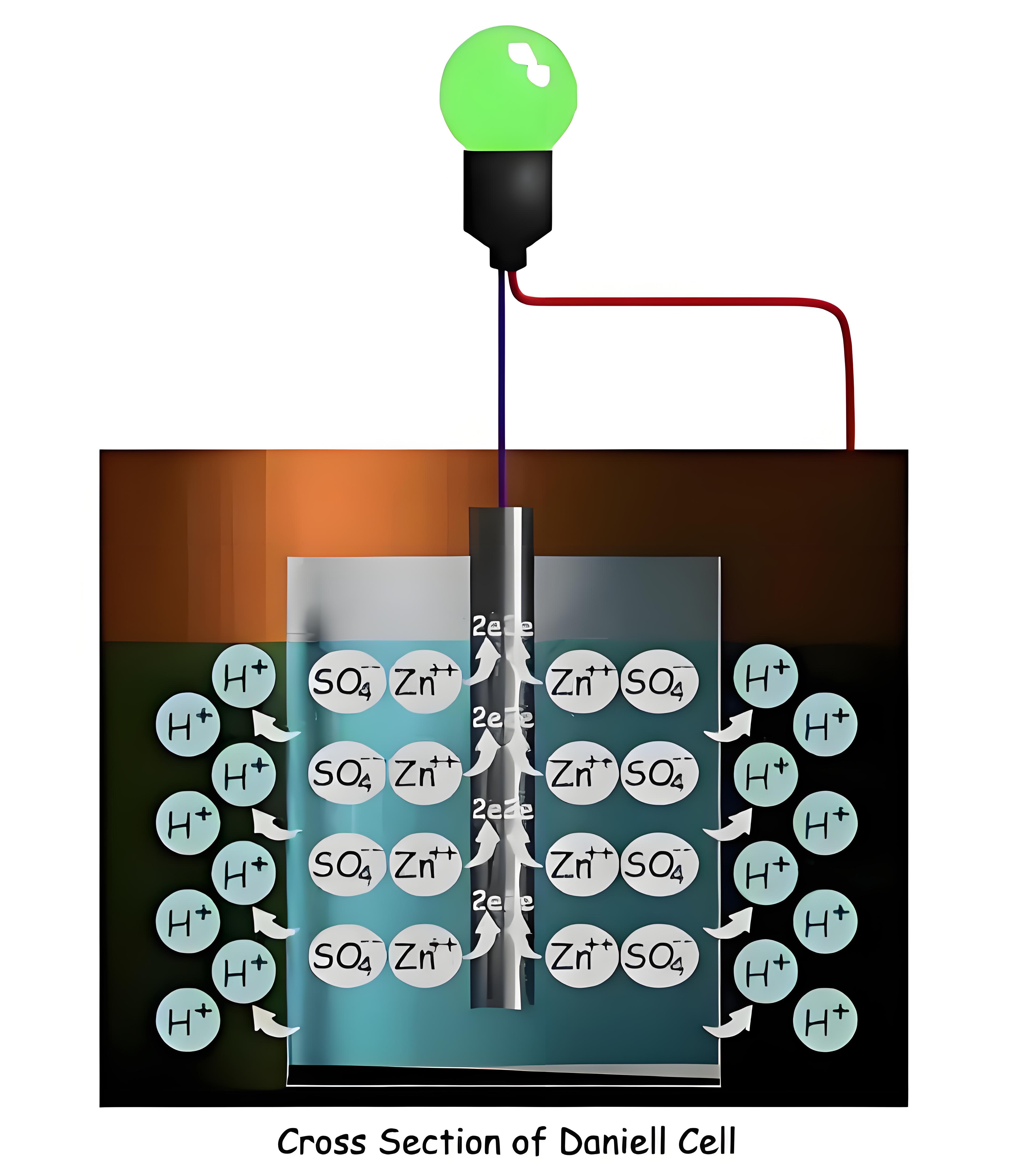
Avoiding Polarization
The Daniell Cell prevents hydrogen gas buildup on the anode by converting it into sulfuric acid, ensuring efficient operation.
Welcome to our electricity community! Established to facilitate the exchange and cooperation in the electricity industry and bridge professionals, enthusiasts, and related enterprises.