Care & Maintenance of Lead Acid Batteries
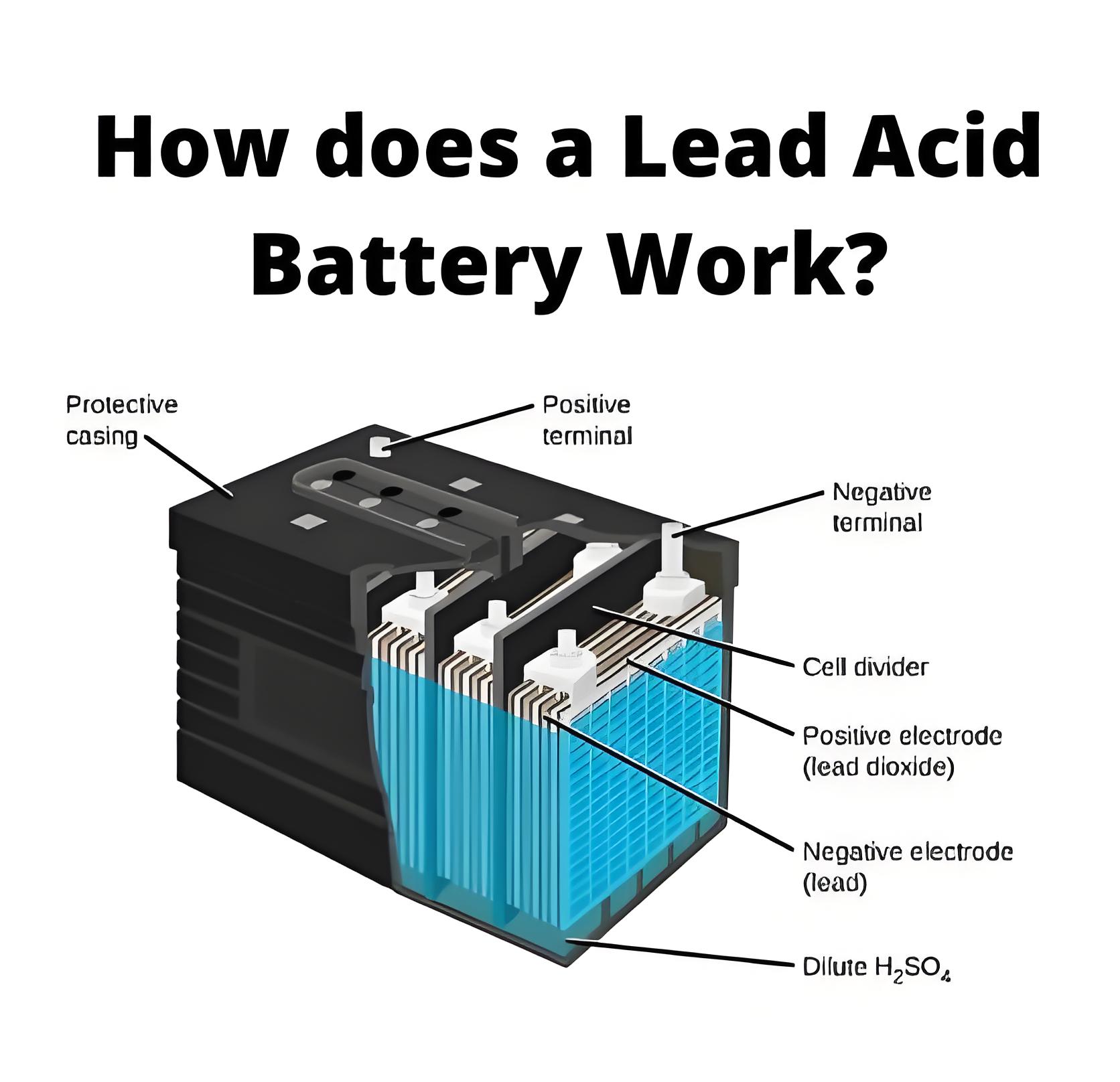
Electrolyte of Lead Acid Battery
The electrolyte of a lead acid battery cell is a solution of sulfuric acid and distilled water. The specific gravity of pure sulfuric acid is about 1.84 and this pure acid is diluted by distilled water until the specific gravity of the solution becomes 1.2 to 1.23. However, in some cases, the specific gravity of diluted sulfuric acid is recommended by the manufacturer of the battery depending upon the type of battery, season, and climatic conditions.
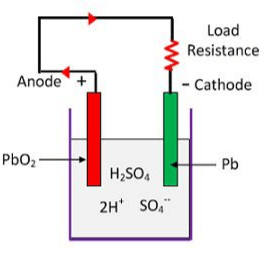
Chemical Action of Lead Acid Battery
The battery cells can be recharged by reversing the direction of discharge current, in the battery. This is done by connecting, the positive terminal of a DC source with the positive terminal of the battery and similarly, the negative terminal of the DC source with the negative terminal of the battery.
(Note: DC stands for “Direct Current”, also called “DC Current“)
A rectifier-type battery charger of suitable capacity is used as a DC source for changing the battery. Due to the charging current (reverse of discharging current) positive plates change to lead peroxide and negative plates change to pure lead.
As soon as the load is connected across the battery terminals, the discharge current starts to flow through the load and the battery starts discharging.
During the discharging process, the acidity of the electrolyte solution decreases, and lead sulfate deposits on both positive and negative plates. In this process of discharge, the amount of water in the electrolyte solution increases and the specific gravity of the electrolyte decreases.
Theoretically, this discharge process continues until the negative and the positive plates contain the maximum amount of lead sulfate, and at that point both types of plate become electrically similar which means there is no potential difference between the electrodes of the cell. But practically, no battery cell is allowed to be discharged up to this point.
The battery cells are allowed to be discharged up to a predetermined minimum cell voltage and specific gravity. A fully charged lead acid battery cell has voltage and specific gravity, of 2.2 V and 1.250 respectively, and this cell is normally allowed to be discharged till the corresponding values become 1.8 V and 1.1 respectively.
Maintenance of Lead Acid Battery
If the cells are overcharged, the physical property of lead sulfate gradually changes, and it may become obdurate from which it becomes difficult to convert by the charging process. Hence, the specific gravity of the electrolyte decreases for which the rate of chemical reaction is hampered.
Sulfated battery cells can easily be recognized by viewing the changed color of plates. The color of a sulfated plate becomes lighter and its surface becomes harsh and gritty. Such cells evolve gas prematurely on charge and show a decreased capacity.
If the sulphation is allowed for a long time, it becomes difficult to rectify the cells. To avoid this situation, it is recommended to charge the lead acid battery cells for a prolonged time at a low rate of charging current.
There is always a high chance that the terminal connectors of the battery cells to be corroded. Corrosion mainly affects the bolted connection between cells in a row. This can easily be avoided if the tightness of each bolt is properly checked and rectified and also each nut bolt connection is covered by a thin layer of petroleum jelly. If any of the cells is corroded, it should be replaced immediately.
The specific gravity of the electrolyte may be permanently decreased due to aging effects. This problem is generally found in old battery cells. This is mainly due to,
The action of sediment at the bottom of the cell container.
Due to loss of acid by spray during charging.
Inadequate treatment after the removal of the short circuit.
Due to excessive sulphation on the plates.
If the lowering of specific gravity is not due to sulphation or a short circuit, concentrated sulfuric acid may be added to restore the normal value of specific gravity.
A short circuit may occur between the positive and negative plates either due to treeing or due to buckling of the plates. Treeing is usually due to excessive gassing which tends to loosen the active materials from the plates.
The particles of active materials fall into the electrolyte and may accumulate on the negative plates in such a way as to bridge the space between the positive and negative plates. This treeing can be removed by the use of a scaling stick made of ebonite.
With this stick, it is possible to explore the space between these two types of plates of a cell and to remove loose materials or treeing.
If the short circuit is due to buckling of plates, this can be removed by inserting an additional separator or by removing and straightening the plates mechanically.
After the removal of the short circuit, care should be taken to restore the specific gravity of the electrolyte to normal by constantly charging by high current.
Maintaining Battery Lead Acid Battery Room
There is a high chance of acid spray and gases during the charging of the battery. These may pollute the atmosphere surrounding the battery. Hence, ample space and good ventilation are essential inside the battery room.
These gases can explode and hence naked flames should not be brought inside the battery room also smoking is strictly prohibited inside the battery room. There should be at least one exhaust fan of suitable size, fitted in the battery room to keep the atmosphere free from those gases and free from moisture inside the room.
The temperature inside the battery room should always be maintained above 10oC. The walls, ceilings, doors, window frames, ventilators, metal parts, and other apparatus in the battery room should be painted with anti-acid coating at regular intervals. The electrical wiring inside the room should be in metal conduit and lighting fixtures should be flameproof in construction.
All the switching elements including electrical fuses and plug sockets should be installed outside the battery room otherwise there may be a chance of fire hazard initiated from sparking during the switching operation. The floor of the room should be well finished preferably by using ceramic tiles. The floor and walls of the room should be cleaned properly at regular intervals.
There are some safety measures to be taken during handling storage batteries in the substation |
|
1 |
Do not smoke inside the room. |
2 |
Do not bring a flame inside the room. |
3 |
Do not generate any spark inside the room. |
4 |
Wear splash-proof goggles, and rubber gloves, while working with the battery. |
5 |
During preparing electrolytes always add the concentrated acid little by little to the water. |
6 |
Never pour water into the concentrated acid. |
All the above-mentioned points should be displayed on the door or any other well-visible place in the battery room.
The following rules are to be obeyed during the operation, control, and emergency lighting service of the storage battery.
Do not allow the battery to stand idle for a long time, this may cause inactivation of the battery cells.
Do not charge the battery with a very high rate of current because the high rate of charging causes high-temperature rise and excessive gassing, resulting in heavy loss of water and sometimes overflowing of electrolytes from the battery cells.
After every complete discharge, the battery should be immediately charged before returning it to its regular floating service. Otherwise, there may be a chance of deposition of sulfate film on the plates.
As already mentioned, the battery cells should be charged gently at a normal rate so that there will be no chance of immediate gassing and temperature rise over 40 oC. Otherwise, there may be damage to the battery cells due to high temperature. During charging of batteries, continuous monitoring is required, if gassing starts and the temperature reaches the said limit, then reduce the rate of charging. If after reducing the charging rate, the temperature is still approaching the limit, it indicates the completion of the charging process because even the normal rate of charging may produce a high-temperature rise if the battery approaches the fully charged condition.
The voltage of each of the battery cells should be checked before the conclusion of charging and each of the cells is properly and equally charged the readings should also be tallied with previous records.
If the electrolyte level inside the battery cell comes down, it must be filled with distilled water up to the level marked on the cell itself. This is to compensate for the loss of water due to evaporation.
During filling up distilled water in the battery cell, it must be carefully watched that the electrolyte level in the battery cells should not exceed the line marked on it. Otherwise, there may be a chance of overflowing of electrolytes during the gassing of the battery. High levels of electrolyte may also cause softening of the sealing compound on the top cover and subsequent leakage in the battery cell.
The specific gravity should be measured after at least two weeks from topping up to ensure a thorough mixing of water in the electrolyte.
The battery should be discharged up to the allowable limit and then it should be overcharged once in 2 to 3 months. The rate of overcharging must be followed as specified by the manufacturer. This operation is very important to maintain the acid storage battery in its active state.
When the specific gravity of the electrolyte is measured, it should not be forgotten to correct it for temperature. So, all hydrometer readings will be referred to the same temperature. The hydrometer should be kept clean with distilled water otherwise the hydrometer will cause incorrect readings and as well as spoil the quality of electrolytes. The specific gravity of the electrolyte must be kept within 1.180 to 1.240. The low value of specific gravity decreases the capacity of the battery and on the other hand high value is harmful to the battery plates.
The substation battery normally operates under floating mode. At floating mode, a constant voltage from the battery charger is applied across the battery during its normal operation to keep the battery approximately in fully charged condition. In normal condition, the battery charger supplies the substation load and also compensate for the losses in the battery. But in case of heavy demand during simultaneous operation of many switch gears, the battery and the charger, are combined to supply the demand.
During normal floating conditions the cell voltage, specific gravity, and temperature of pilot cells should be measured daily to follow up on the condition of the battery as a whole. However, the same readings of each and every battery cell should be taken at least once a month to keep monitoring the conditions of individual cells.
The battery should be refilled with fresh electrolytes once in three-year intervals to maintain the battery in proper operating conditions.
It should be noted that the pilot cell of a battery bank is one arbitrarily chosen battery cell that is used to get information about the overall condition of the battery. But one pilot cell must be fixed for one month and it should be changed in the next month.
This was a brief description of the maintenance of the substation battery but it is always preferable to follow the instructions given in the maintenance manual supplied by the manufacturer too.
Statement: Respect the original, good articles worth sharing, if there is infringement please contact delete.
Welcome to our electricity community! Established to facilitate the exchange and cooperation in the electricity industry and bridge professionals, enthusiasts, and related enterprises.