Lamp Materials: A Comprehensive Guide
A lamp is a device that produces illumination by using a wick soaked in combustible material or other light-producing instruments such as gas and electric lamps. Lamps were invented at least as early as 70,000 BCE and have evolved over time to use different materials and designs. In this article, we will explore the various types of materials that are used to build a lamp, and their properties and functions.
What is a Lamp Material?
A lamp material is any substance that is used to construct a lamp or its components. Lamp materials can be classified into two main categories: insulating materials and conducting materials. Insulating materials are those that do not allow an electric current to pass through them, such as glass, ceramics, and plastics. Conducting materials are those that allow electric current to flow through them, such as metals and alloys.
Insulating materials are used to form the barrier or the enclosure of the lamp, which protects the light source from external factors and influences the color and quality of the light. Conducting materials are used to form the filament, the electrode, the lead-in wire, and the base or the end cap of the lamp, which provide the electrical connection and support for the light source.
Types of Lamp Materials
There are many types of lamp materials that are used for different purposes and applications. Some of the most common ones are:
Glass
Glass is a transparent material that is made from melted sand or silica mixed with other substances. Glass is widely used as a barrier or an enclosure for lamps, as it can withstand high temperatures and pressures and can be shaped into various forms and colors. Glass can also transmit light with minimal loss or distortion, and can be chemically inert and resistant to corrosion.
Some of the types of glass that are used for lamps are:
Soda-lime silicate glass: This is the most common type of glass, which has a low melting point and is used for filament lamps. It contains about 67% silica, along with sodium oxide, calcium oxide, and other additives.
Lead-alkali silicate glass: This is a type of glass that has a higher electrical resistivity than soda-lime glass, and is used for the inner portion of the bulb glass. It contains lead oxide, potassium oxide, and other additives.
Borosilicate glass: This is a type of glass that has a higher temperature resistance and a lower thermal expansion coefficient than soda-lime glass and is used for higher-wattage lamps, such as cinema projectors. It contains boron oxide, aluminum oxide, and other additives.
Alumina silicate glass: This is a type of glass that has a lower thermal shock resistance than borosilicate glass but a higher refractive index and is used for low-wattage lamps with high light output. It contains alumina, magnesia, and other additives.
Quartz: This is a type of glass that is made from pure silica or silicon dioxide, which has a very high melting point and transparency. It is used for tungsten halogen lamps, which operate at very high temperatures. It contains only trace amounts of other metals and hydroxyl groups.
Sodium-resistant glass: This is a type of glass that is specially designed for sodium vapor lamps, which produce intense light by ionizing sodium vapor. Sodium vapor has a powerful reducing property that can cause rapid blackening of normal glasses. The sodium-resistant glass contains small amounts of silica or other readily reducing oxides to prevent this effect.
Ceramics
Ceramics are non-metallic materials that are made from clay or other inorganic substances that are heated and hardened. Ceramics are used for lamps because they can be molded into various shapes and sizes and can have different optical properties, such as transparency or translucency. Ceramics can also withstand high temperatures and pressures and can be chemically stable and resistant to corrosion.
Some of the types of ceramics that are used for lamps are:
Polycrystalline metal oxide ceramics: These are ceramics that are made from metal oxides such as alumina, magnesia, or rare earth oxides, which are heated and sintered to form polycrystalline bodies. These ceramics can be transparent or translucent depending on their porosity and grain size. They are used for high-pressure lamps such as sodium vapor lamps or metal halide lamps, which require high light transmission.
Conventional ceramics: These are ceramics that are made from clay or other natural substances that are mixed with water and shaped into desired forms before firing. They include porcelain and steatite.
Porcelain: This is a type of ceramic that is made from kaolin clay mixed with feldspar, quartz, and other additives. It has good mechanical strength, thermal shock resistance, electrical insulation property, and moisture resistance. It is used to make bases or end caps for lamps.
Steatite: This is a type of ceramic that is made from talc mixed with clay and other additives. It has better properties than porcelain in terms of electrical resistivity, thermal conductivity, dielectric strength, and dimensional stability. It is used to make insulators or supports for lamps.
Metal
Metal is an element or an alloy that has high electrical conductivity and thermal conductivity. Metal is used for lamps because it can provide electrical connection and support for the light source, as well as reflect or diffuse light depending on its surface finish. Metal can also be shaped into various forms and sizes by casting, forging, machining, or welding.
Some of the types of metal that are used for lamps are:
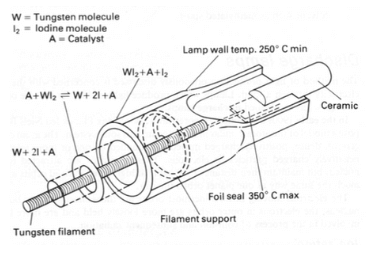
Tungsten: This is an element that has a very high melting point (3422°C) and tensile strength (1510 MPa). It is used to make filaments for incandescent lamps by drawing it into thin wires and coiling them around iron or molybdenum mandrels. Tungsten filaments have high resistance to heat and evaporation, but they also require high voltage to operate.
Molybdenum: This is an element that has a high melting point (2610°C) but lower tensile strength (638 MPa) than tungsten. It is used to make supports or lead-in wires for filaments, as well as electrodes for arc lamps. Molybdenum has a similar coefficient of expansion to some types of glass, which allows it to form tight seals with them.
Nickel: This is an element that has a moderate melting point (1455°C) and tensile strength (758 MPa). It is used to electroplate iron or steel components to increase their hardness and elasticity. Nickel also has high resistance to corrosion and oxidation. It is used to make lead-in wires or bimetallic strips, for starters.
Aluminum: This is an element that has a low melting point (660°C) but high tensile strength (310 MPa). It is also lightweight (2.7 g/cm3) and non-magnetic. It has high corrosion resistance due to the thin oxide layer on its surface. Aluminum is easily available and cheap in price. It is used to make caps or reflectors for lamps.
Steel: This is an alloy of iron with carbon and other elements such as manganese or chromium. Steel has a variable melting point (1370°C – 1530°C) depending on its composition but high tensile strength (400 MPa – 2000 MPa). Steel also has good ductility and malleability. Steel sheet has high strength but low cost compared to other metals. Steel sheets can be hot-rolled or cold-rolled, depending on their thickness and surface finish. Steel sheets can also be coated with porcelain enamel to improve their appearance or corrosion resistance.
Stainless steel: This is an alloy of iron with chromium (12% – 30%) and other elements such as nickel or molybdenum. Stainless steel has high corrosion resistance due to its chromium oxide layer on its surface. Stainless steel also has good mechanical properties such as strength (515 MPa – 1035 MPa), hardness (95 HRB – 40 HRC), ductility (45% – 60%), toughness (100 J – 225 J), fatigue resistance (275 MPa – 690 MPa), creep resistance (35 MPa – 200 MPa), wear resistance (0.04 g – 0.4 g), abrasion resistance (0.2 mm – 1 mm), erosion resistance (0.02 mm – 0.2 mm), cavitation resistance (0 mm – 0.05 mm), pitting resistance (0 mm – 0 mm), stress corrosion cracking resistance (0 mm – 0 mm), intergranular corrosion resistance (0 mm – 0 mm), galvanic corrosion resistance (0 mV – +50 mV), fretting corrosion resistance (0 mg – <1 mg), hydrogen embrittlement resistance (>100 MPa), sulfide stress cracking resistance (>100 MPa), carburization resistance (>100 MPa), nitriding resistance (>100 MPa), oxidation resistance (>1000°C), sulfidation resistance (>800°C), carburization resistance (>800°C), nitriding resistance (>800°C), decarburization resistance (>800°C), scaling resistance (>800°C), spalling resistance (>800°C), embrittlement resistance (>800°C), and thermal shock resistance (>800°C). Stainless steel is used for luminaires, especially outdoor ones, where there is a chance of exposure to corrosive atmospheres.
Copper: This is an element that has high electrical conductivity (59.6 MS/m) and thermal conductivity (401 W/mK). Copper is also ductile and malleable and can be easily shaped into various forms. Copper is used for conductors, such as bus bars, switch gears, and lead-in wires, as well as electrodes for arc lamps. Copper also has good corrosion resistance, especially against seawater.
Non-ferrous alloys: These are alloys that do not contain iron as a major component, such as bronze, brass, or solder.
Bronze: This is an alloy of copper and tin, with varying proportions of other elements such as zinc or phosphorus. Bronze has good mechanical properties, such as strength (200 MPa – 1200 MPa), hardness (60 HB – 250 HB), ductility (3% – 40%), and toughness (25 J – 200 J). Bronze also has good corrosion resistance, especially against seawater and acidic solutions. Bronze is used for special luminaires that have an attractive color appearance.
Brass: This is an alloy of copper and zinc, with varying proportions of other elements such as lead or nickel. Brass has good mechanical properties, such as strength (200 MPa – 900 MPa), hardness (50 HB – 200 HB), ductility (10% – 50%), and toughness (30 J – 150 J). Brass also has good corrosion resistance, especially against seawater and alkaline solutions. Brass is used for special luminaires that have an attractive color appearance.
Solder: This is an alloy of tin and lead, with varying proportions of other elements such as silver or antimony. Solder has a low melting point (183°C – 232°C) and high wettability, which means it can adhere to metal surfaces easily. Solder is used to join metal components together by melting and solidifying them. Solder is used at the end of the lamp cap for electrical connection.
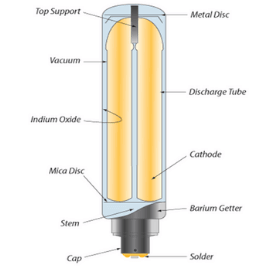
Getter material: This is a material that is used to absorb the gas impurities that are produced inside the lamp during operation, as they can lower the lamp’s performance. The gas impurities include oxygen, carbon monoxide, carbon dioxide, nitrogen, hydrogen, water vapor, and others. Getter material can be in the form of sheet, wire, or surface deposit and can be activated by heating or exposure to ultraviolet light. Some of the getter materials that are used for lamps are:
Barium: This is an element that has a high affinity for oxygen and nitrogen and can form stable compounds with them. Barium is used as a metallic getter material for incandescent lamps and fluorescent lamps.
Tantalum: This is an element that has a high affinity for oxygen and nitrogen and can form stable compounds with them. Tantalum is used as a metallic getter material for tungsten halogen lamps and metal halide lamps.
Titanium: This is an element that has a high affinity for oxygen and nitrogen and can form stable compounds with them. Titanium is used as a metallic getter material for sodium vapor lamps and mercury vapor lamps.
Niobium: This is an element that has a high affinity for oxygen and nitrogen and can form stable compounds with them. Niobium is used as a metallic getter material for sodium vapor lamps and mercury vapor lamps.
Zirconium: This is an element that has a high affinity for oxygen and nitrogen and can form stable compounds with them. Zirconium is used as a metallic getter material for sodium vapor lamps and mercury vapor lamps.
Barium-tantalum-titanium alloy: This is an alloy of barium, tantalum, and titanium that has a high affinity for oxygen and nitrogen and can form stable compounds with them. This alloy is used as a metallic getter material for sodium vapor lamps and metal halide lamps.
Red phosphorus: This is a non-metallic element that has a high affinity for oxygen and water vapor and can form stable compounds with them. Red phosphorus is used as a non-metallic getter material for incandescent lamps and fluorescent lamps.
Conclusion
Lamp materials are substances that are used to construct lamps or their components. They can be classified into insulating materials and conducting materials, depending on their electrical properties. Insulating materials are used to form the barrier or the enclosure of the lamp while conducting materials are used to form the electrical connection and support for the light source. Some of the common lamp materials are glass, ceramics, metal, and getter materials. Each of these materials has different properties and functions that affect the performance and appearance of the lamp. By understanding the types and characteristics of lamp materials, one can choose the best material for a specific lamp application or design.
Statement: Respect the original, good articles worth sharing, if there is infringement please contact delete.
Welcome to our electricity community! Established to facilitate the exchange and cooperation in the electricity industry and bridge professionals, enthusiasts, and related enterprises.