Mechanism of Polarization
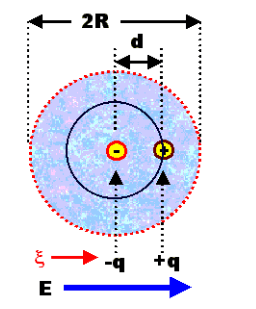
First of all we have to know the definition of polarization before entering into mechanism. Polarization is actually the alignment of the dipole moments of the fixed or induced dipole in the direction of the peripheral electric field. The mechanism of polarization deals with how a molecule or atom is reacting to a peripheral electric field. Simply we can say that it leads to the positioning of dipoles.
There are fundamentally four divisions of polarization mechanisms. They are Electronic polarization, dipolar or Orientation polarization, Ionic polarization and Interfacial polarization. Let us discuss the different polarization in detail.
Electronic Polarization
Here, the neutral atoms get polarized and it results in the shifting of electrons. It is also known as atomic polarization. We can simply say that with respect to the nucleus, the center of electrons is shifted. Hence, a dipole moment is formed as represented below.
Orientation Polarization
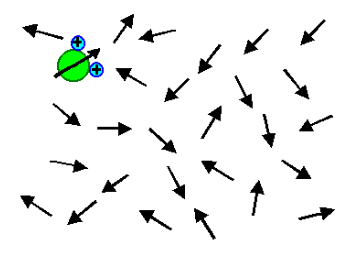
It is also known as dipolar polarization. Due to the thermal equilibrium of the molecules, in normal state the dipoles will be randomly aligned. When a peripheral electric field is implemented, it results in polarization. Now, the dipoles will align to some degree as represented in figure 2. E.g.: It usually occurs in gases and liquids such as H2O, HCl etc.
Ionic Polarization
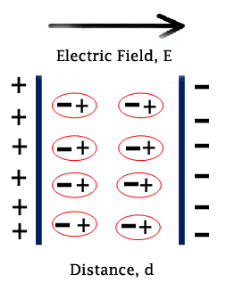
From the name itself we can say that it is the polarization of ions. It results in the shifting of ions and forms dipole moment. It usually occurs in solid materials. E.g: NaCl. In normal state, it contains some dipoles and they nullify each other. It is represented in figure 3.
Interfacial Polarization
It is also known as space charge polarization. Here, due to the peripheral electrical field, at the interface of electrode and material the orientation of charge dipoles takes place. That is; when a peripheral electric field is implemented, movement of some positive charges to the grain boundary takes place and result in the assemblage. It is shown in figure 4.
However, in most of the cases more than one polarization will be present in one material. Electronic polarization happens in almost all materials. So for us, the dielectric characterisation of real materials can be really difficult. For finding the total polarization, we will consider all the other polarizations except interfacial polarization. The reason is that, we have no method for computing the charges present in interfacial polarization.
When we go through the four polarization mechanism, we can see that the volume of the drifted entities is different for each of them. It can be seen that the gradual increase in mass happens from electronic to orientation polarization. The frequency of the peripheral electric field has direct relation with these mass. So we can conclude that, when mass to be drifted increases, the time for drifting it also increases.
Next, we can discuss about how the dielectric constant of the non-magnetic dielectrics which comes from the electrical part is connected to refraction index (at high frequency 1012-1013 Hz). It is by
For example C (Diamond) has


Statement: Respect the original, good articles worth sharing, if there is infringement please contact delete.
Welcome to our electricity community! Established to facilitate the exchange and cooperation in the electricity industry and bridge professionals, enthusiasts, and related enterprises.