Orientational Polarization
Before discussing Orientational polarization, let us examine the structural details of some molecules. Let us take an oxygen molecule. A single oxygen atom has only 6 electrons on its outermost cell. One oxygen atom creates the double covalent bond with another oxygen atom and creates an oxygen molecule. In an oxygen molecule, the distance between the centers of the nucleus of two atoms is 121 Pico-metre. But there is no permanent or resultant dipole moment as both ends of the molecules are equally charged. There is no net charge transfer between the atoms in the molecule. Similarly, if we take the pictures of hydrogen, nitrogen etc we will find there is also no net dipole moment for the same reasons. Now, let us consider the molecular structure of water.
A water molecule is bent structured. Here, oxygen atom has the covalent bond with two hydrogen atoms. Oxygen portion of the water molecule is slightly negative whereas hydrogen portions are slightly positive. These negative positive portions of the molecules form two dipole moments pointed from the center of oxygen atom to center of hydrogen atoms.
The angle between these two dipole moments is 105o. There would be a resultant of these two dipole moments. This resultant dipole moment is present in each of the water molecule even in the absence of any externally applied field. So, the water molecule has a permanent dipole moment. Nitrogen dioxide or similar types of molecules have same permanent dipole moment for same reason.
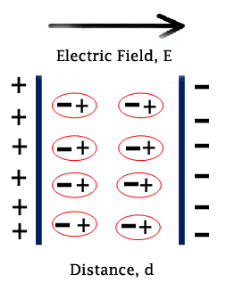
When an electric field is applied externally, the molecules with permanent dipole moment orient themselves according to the direction of applied electric field. This is because external electric field exerts a torque on the permanent dipole moment of each molecule. The process of orientation of permanent dipole moments along the axis of applied electric field is called orientational polarization.
Statement: Respect the original, good articles worth sharing, if there is infringement please contact delete.
Welcome to our electricity community! Established to facilitate the exchange and cooperation in the electricity industry and bridge professionals, enthusiasts, and related enterprises.