Ionic Polarization
Ionic Polarization
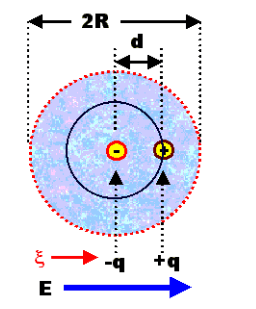
Before understanding what ionic polarization let is us observe how a sodium chloride (NaCl) molecule is formed. Sodium chloride (NaCl) molecule is formed by ionic bond between sodium and chlorine atoms. The sodium atom gives up one electron to get eight electrons in its outer most orbits. In this way the sodium atom becomes positive ion. On the other hand chlorine atom takes one electron to make eight electrons it its outermost orbits and becomes negative ion. Now due to electrostatic force between positive sodium and negative chlorine ions, they bound together and form sodium chloride molecule. Naturally, each of the sodium chloride molecules has a positive end and a negative end. Because, sodium portion of the molecule will be slightly positively charged due to presence of positive sodium ion and chlorine side will be slightly negatively charged due to presence of negative chlorine ion.
As there is an inter nucleus distance in the sodium chloride molecule, there must be a dipole moment present in the molecule even in absence of any externally applied electric filed. As the sodium chloride molecules have only two atoms (ions) there must be a single dipole moment pointing from negative to positive ion in each molecule. But there are many ionic compounds which have more than two atoms. In these cases, there will be more than one ionic bond and hence there must be dipole moments as many as the number of bonds in a molecule. But all the dipole moments are directed from relatively negative ion to positive ion. The resultant dipole moment of a single molecule would be the vector sum of individual dipole moments of the molecule.
If the molecule has center of symmetry, then the molecule may have number of inter ionic dipole moment but resultant over all dipole moment of the molecule would be zero. Net dipole moment of the molecule presents only in asymmetrical structure of molecules. This net dipole moment of the molecule is referred as permanent dipole moment as this presents in the molecule even in absence of any peripheral electric field. Let us take the reference of the following figures. In the first figure the molecule is made of two atoms and it has only single dipole moment directed from negative to positive ions. In the figure 2, the molecule has center of symmetry.
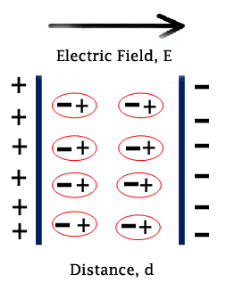
There are two dipole moments from negative to positive ions but they cancel each other. So there is on net dipole moment of the molecule. In figure 3, there is a net dipole moment because of asymmetrical structure of molecule. So the molecules may have permanent dipole moment or not but as soon as an external electric field is applied, the negative ions of the molecules will tend to shift toward positive side of the applied field and positive ions of the molecules will tend to shift towards negative side of the applied electric field.
This is called ionic polarization. If there are N number of polarized molecules present in unit volume of the material. The ionic polarization of the material is given by
Where, µionic is the average induced dipole moment of the molecule due to externally applied electric field. This is obviously proportional to the strength of applied electric field. So,
Again, when external field is applied there will be slight shifting of positive nucleus and negative electrons of each atom of the molecules. Because of that there will be an electronic dipole moment in each atom of the molecules. This electronic dipole moment is also proportional to the number of molecules per unit volume and strength of applied electric field. The proportionality constant or polarizability for that say, α electronic.
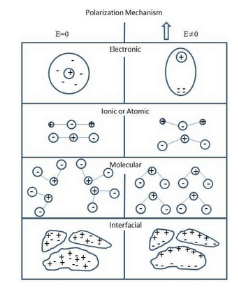
It is needless to say that whenever an electric field is applied in a dielectric of ionic compound, there would be two types of polarization occur in it. These are ionic polarization and electronic polarization. The total polarization is the sum of these two polarizations.
Statement: Respect the original, good articles worth sharing, if there is infringement please contact delete.
Welcome to our electricity community! Established to facilitate the exchange and cooperation in the electricity industry and bridge professionals, enthusiasts, and related enterprises.